The Common Vein Copyright 2010
Introduction
Prostate carcinoma is a malignant disease usually originating in the secretory cells of the prostate glands, often multicentric in location. It is the most commonly diagnosed cancer in men and the second leading cause of cancer death in men second to lung cancer.
Tumors of the prostate are estimated to be found in about half of the men in their 70’s and almost all men in their 90’s. The incidence of prostate cancer differs among different ethnicities. The incidence is highest among African Americans and lowest among Asian Americans.
Structurally, it can present with a focal thickening in the periphareral zone, along with irregularities in the prostate contour.
Functionally there is limited impairment, and diagnosis is often made incidentally or during screenings.
Complications include early spread to the regional nodes and spread to distal areas, such as the skeleton.
Clinically the patient is usually asymptomatic with localized disease. With progression patients may experience bony pain, urinary symptoms from obstruction, or hematuria. Survival rates vary depending on how aggressive the disease is at the time of diagnosis.
The diagnosis is suspected by DRE showing a nodule or induration, as well as and elevated PSA, but is often found incidentally at autopsy. As such it is a highly heterogeneous disease with varying degrees of impact on morbidity and mortality. Biopsy is the gold standard for diagnosis. It is believed that screening has played a significant role in reducing mortality from prostate cancer. Screening utilizes various measurements of PSA levels.
From an imaging standpoint, ultrasonography can reveal a hyperechoic, hypoechoic, or isoechoic leasion. Endorectal T2-weighted MRI may be more specific. CT is of little use, though contrast-enhanced CT may demonstrate enhancement in tumors. Bone scintigraphy may be peformed to evaluate for osseous metastases.
Treatment of prostate cancer depends on several factors, namely the grade and stage of the tumor, life expectancy of the patient, and the ability to ensure disease-free survival. Surgical treatment provides the only opportunity for cure and is indicated in the presence of aggressive disease (Gleason 7-10), or dependent on the age at diagnosis. Gleason 6 is considered by many to be non-operative but should undergo close surveillance. The role of neoadjuvant therapy prior to surgery is indicated in patients with T3 disease, followed by external beam radiotherapy. If a resection results in positive tumor margins, adjuvant radiotherapy may also be indicated.
Metastasis
Prostate spreads primarily by contiguous spread locally via lymphatic vessels to regional lymph nodes, as well as hematogenously to distant sites. Initially spread to distant sites is mostly within the axial skeleton. In later stages of disease spread to the lungs, liver, and kidneys may also occur.
Bone
Spread to bones is common, spread of disease to the bones is often monitored via bone scintigraphy. While new foci of disease may be seen, the resulting blastic activity is not particularly effective in demonstrating a response to therapy.
|
Zonal Anatomy of the Prostate in Transverse Plane |
|
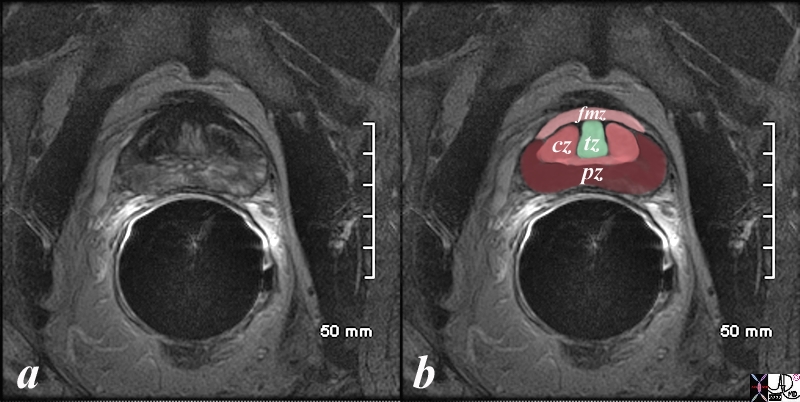
The prostate gland can be viewed as having 4 major zones. In the young adult male the outer zone called the peripheral zone, accounts for 70% of the parenchyma. Inward of the peripheral zone is the central zone that accounts for 25% of the parenchyma. The periurethral zone is called the transitional zone and fibromuscular layer i(anterior layer account for the remaining 5% of parenchyma Courtesy Ashley Davidoff MD Copyright 2010 25078b04b02L05L.8s |
|
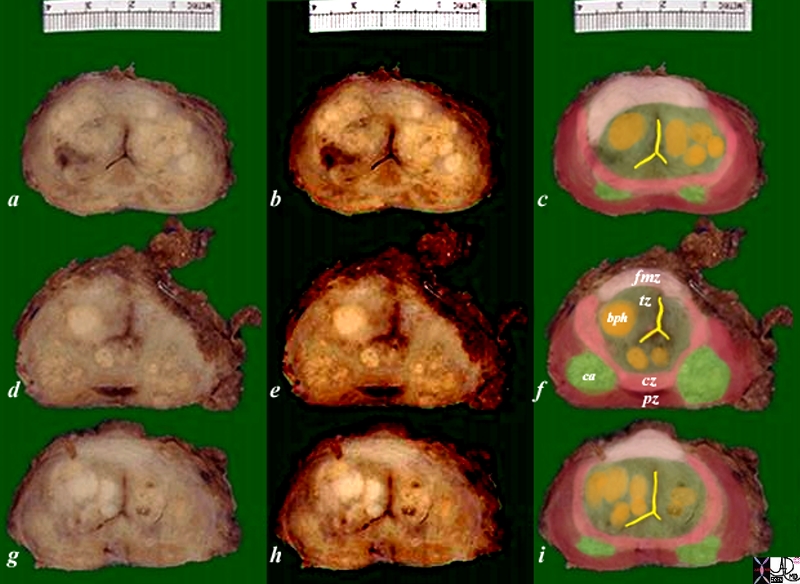
Zonal Anatomy and Predilections For Carcinoma – Peripheral Zone |
|
The diagram depicts the zonal morphology relevant to the locations where disease arises. The normal gland is seen in image (a). The prostate gland can be viewed as having 4 major zones. The outer zone is called the peripheral zone, accounts for 70% of the parenchyma. Inward of the peripheral zone is the central zone that accounts for 25% of the parenchyma. The periurethral zone is called the transitional zone and fibromuscular zone (anterior layer) account for the remaining 5% of parenchyma. Image (b) shows BPH (benign prostatic hypertrophy) which is caused by hyperplasia and enlargement of the transitional zone. Image (c) shows carcinoma (bright lime green) arising from the peripheral zone which is the characteristic site of origin of this disease. Image (d) shows a combination of BPH in the transitional zone and carcinoma in the peripheral zone. This is a common combination of diseases in the elderly population. Image Courtesy Ashley Davidoff MD Copyright 2010 25078cL01.8s |
|
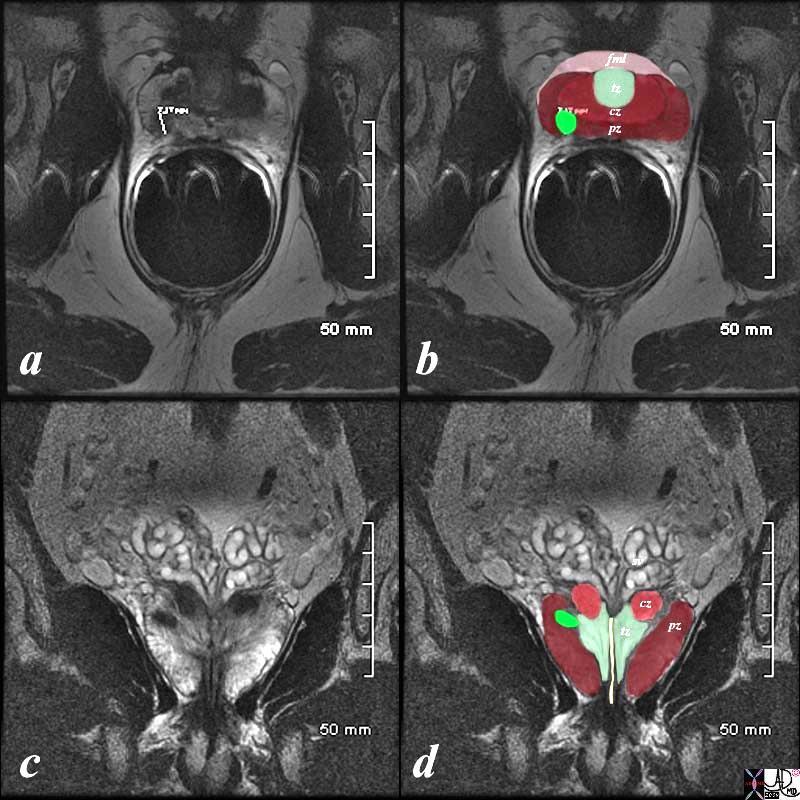
Normal Zonal Anatomy MRI T2 Weighted |
|
The patient is a 60 year old man. His MRI was performed with a transrectal coil and the image shows the T2 weighted sequence in the axial projection (a,b). The scan shown demonstrates the normal zonal anatomy of the prostate. The prostate gland can be viewed as having 4 major zones. In the normal gland the outer zone called the peripheral zone, accounts for 70% of the parenchyma. (maroon, pz). It is often canoe shaped in the axial projection. Inward of the peripheral zone is the central zone (dark pink, cz) that accounts for 25% of the parenchyma. The remaining 5% of the parenchyma is composed of the periurethral zone called the transitional zone (light green), and the fibromuscular zone (light pink, fmz) – the anterior most layer. Image Courtesy Ashley Davidoff MD Copyright 2010 98877c.8s |
|
Prostate Carcinoma MRI T2 Weighted |
|
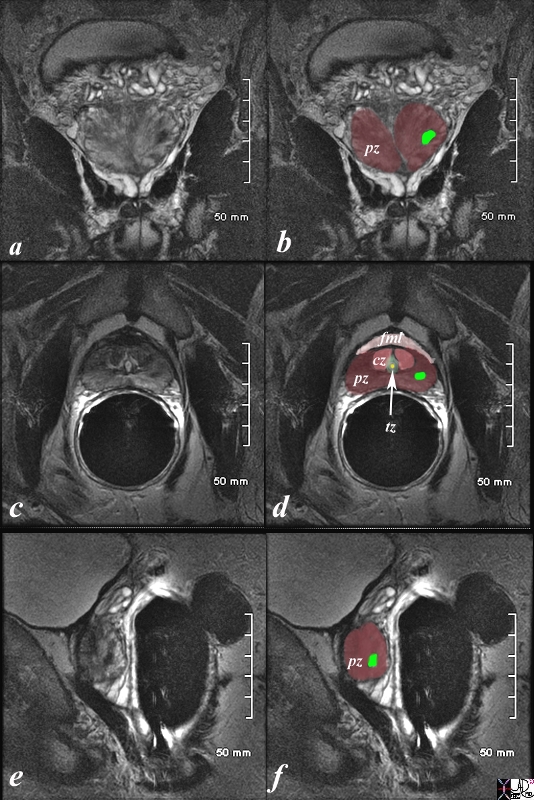
The patient is a 61 year old man with primary prostate carcinoma. His MRI was performed with a transrectal coil. T2 weighted images in the coronal projection (a,b), axial projection (c,d), and sagittal projection (e,f) are shown. The primary lesion is a 8mm lesion ( bright green overlay in b,d,f) is hypointense on the T2 weighted image and noted in left sided peripheral zone (pz darker maroon b,d,f). Other zones noted include central zone (lighter red, cz, in d) transitional zone (pale green,white arrow, tz, in d) and fibromuscular layer (light pink, fbl in d). The urethra is shown in yellow in the middle of the tz in d. The lesion was biopsied and shown to be an adenocarcinoma of the prostate. Image Courtesy Ashley Davidoff MD Copyright 2010 98899cL02.8s |
Right Peripheral Zone MRI T2 Weighted – Endorectal Coil |
|
The patient is a 60 year old man with primary prostate carcinoma. His MRI was performed with a transrectal coil and T2 weighted images in the axial projection (a,b) and coronal projection are shown. The primary lesion is a 8mm lesion ( bright green overlay in b and d) is hypointense on the T2 weighted image (a, b) and noted in the right sided of the peripheral zone (pz darker maroon). Other zones noted include central zone (lighter maroon, cz, in b,d) transitional zone (pale green, tz, in b,d) and fibromuscular layer (light pink, fbm in b) The lesion was biopsied and shown to be an adenocarcinoma of the prostate. Image Courtesy Ashley Davidoff MD Copyright 2010 99320cL.8s |
Carcinoma and BPH |
|
The pathology specimen is from a patient with prostate carcinoma and benign prostatic hyperplasia. Image Courtesy Alexander Perepletchikov MD PhD 99379c03L.8s |
Diagnosis
The detection of small and well-differentiated cancers is often clinically insignificant, since most of these tumors would not manifest clinically and remain latent for the patients entire lifetime.
The main issue of prostate cancer is not in the diagnosis but the potential for excessive trteatment because the disease has the name cancer to it. How does one differentiate between the disease that will have an aggressive natural history and a latent natural history?
According to Stamey et al a tumor that has a volume of less than .5ccs and a Gleason score of less than 7 are more likely to remain latent.
Priority should therefore be given to the histology, staging, and if treatment is considered prudent to use limited focal ablative techniques. Watchful waiting on what appears to be a latent bystander should be supported.
PSA
The role of any screening is to provide a cost-effective means of detecting disease with a relatively long so-called dormant phase at a point when it can effectively be treated and change clinical outcomes.
PSA or Prostate Specific Antigen is a 240 amino acid single chain intracellular glycoprotein. It plays a role in liquefying the semen that is formed after ejaculation. It has a half biologic half-life of approximately 2-3 days.
There is considerable debate regarding the role of PSA in screening for prostate cancer. PSA can be elevated in a variety of situations, including prostate cancer, BPH, and prostatitis. The sensitivity of PSA is about 80%, but the specificity of PSA in detecting prostate cancer is only 65%. In order to improve the sensitivity of PSA in diagnosing prostate cancer, many have utilized PSA velocity, PSA density, and free PSA.
Velocity: Several studies suggest that an increase of PSA greater than 0.75g/year increases the chance of prostate cancer. However, since PSA is highly variable, three separate reading about months apart from each other are needed.
Density: PSA is increased as the prostate gland enlarges. Therefore, higher PSAs in smaller glands may be a sign of cancer. Some use a cutoff of 0.15g/mL3.
Free PSA: Men with prostate cancer have lower levels of free PSA. if a 25% free PSA cutoff is used, 90% of all prostate cancers can be detected.
Gleason Grading
Introduction
It is estimated that if biopsied, 50% of men aged 75 or over will have prostate cancer. In most cases, it is a disease that will not have any clinical impact on life expectancy. Nevertheless, very aggressive forms of prostate cancer exist that can very rapidly result in a patient’s demise. For this reason, it is important to differentiate those cancers that merit treatment, or close active surveillance.
The Gleason scoring system is a means of histologically grading the degree of differentiation. Grade 1 disease is well differentiated, while 5 is anaplastic (poorly differentiated). As multiples samples are typically taken at the time of biopsy or resection, the score of a primary and secondary sample are combined for a total score between from 2 to 10.
Grading
The first step in Gleason grading is to distinguish between a well differentiated tumor (grade 1) to poorly differentiated (grade 5).
Gleason grade 1 – closely resembles normal prostate, closely packed, uniform round glands arranged in a circumscribed nodule with pushing borders, very uncommon, never seen in needle biopsies.
Gleason grade 2 – closely resembles normal prostate but more variability in glands shape, acini loosely aggregated and no infiltration of stroma, uncommon, never seen in needle biopsies.
Gleason grade 3 – most common grade by far and is also considered moderately differentiated so that the glandular unit is recognized. Every cell is part of a circular row which forms the lining around the lumen which may contain secretions and are separated by stromal tissue. The glands start to have a random arrangement. Basal cells are absent as in any malignant prostatic gland. Some of these glands invade the stroma which is a differentiating feature of Grade 3. The shapes of the glands are less uniform and the cells are more amphophilic.
Gleason Grade 4 is distinctly different from Grade 3 and it is one of the common problems that pathologists experience to distinguish between these two common grades. Its presence worsens the prognosis. The glands lose their ability to form discrete prostate units and are fused between each other. Cribriforming glands and acinar “glomerulation” seen.
Gleason Grade 5 is less common than Grade 4. Glandular formation is absent. The neoplastic cells identified as cell sheets or isolated cells. It is considered the undifferentiated form of prostate cancer.
The combined Gleason score is a method to combine histological grades and then to name the dominant pattern first and the secondary pattern.
For example, a score of 4+ 3 describes pathology of cancer where both Grade 3 and 4 are present, but the dominant pattern is 4. The combined Gleason score is therefore 7.
Clinical Signficance
Although there is considerable debate, Gleason 6 can be followed with active surveillance, depending on disease burden, while Gleason 7-10 are generally considered aggressive, requiring surgery and possible adjuvant thereapy.
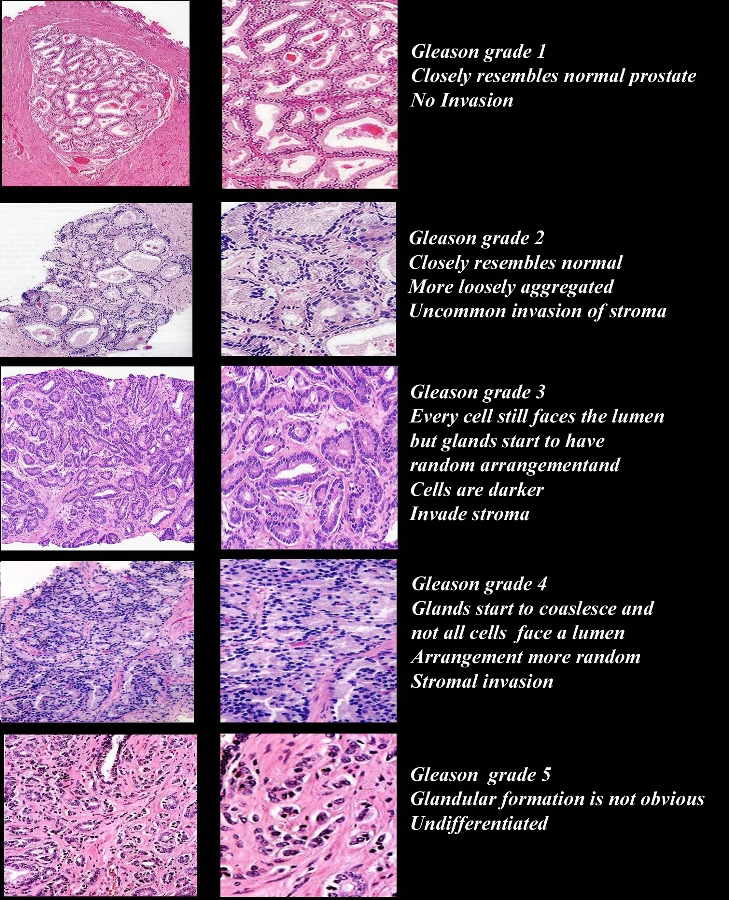
Gleason Grading System |
| The first step in Gleason grading is to distinguish between a well differentiated tumor (grade 1) to poorly differentiated (grade 5). Gleason grade 1 – closely resembles normal prostate, closely packed, uniform round glands arranged in a circumscribed nodule with pushing borders, very uncommon, never seen in needle biopsies. Gleason grade 2 – closely resembles normal prostate but more variability in glands shape, acini loosely aggregated and no infiltration of stroma, uncommon, never seen in needle biopsies. Gleason grade 3 – most common grade by far and is also considered moderately differentiated so that the glandular unit is recognized. Every cell is part of a circular row which forms the lining around the lumen which may contain secretions and are separated by stromal tissue. The glands start to have a random arrangement. Basal cells are absent as in any malignant prostatic gland. Some of these glands invade the stroma which is a differentiating feature of Grade 3. The shapes of the glands are less uniform and the cells are more amphophilic. Gleason Grade 4 is distinctly different from Grade 3 and it is one of the common problems that pathologists experience to distinguish between these two common grades. Its presence worsens the prognosis. The glands lose their ability to form discrete prostate units and are fused between each other. Cribriforming glands and acinar “glomerulation” seen. Gleason Grade 5 is less common than Grade 4. Glandular formation is absent. The neoplastic cells identified as cell sheets or isolated cells. It is considered the undifferentiated form of prostate cancer. The combined Gleason score is a method to combine histological grades and then to name the dominant pattern first and the secondary pattern. For example, a score of 4+ 3 describes pathology of cancer where both Grade 3 and 4 are present, but the dominant pattern is 4. The combined Gleason score is therefore 7. Courtesy Alexander Perepletchikov, MD,PhD 98085c04.9 |
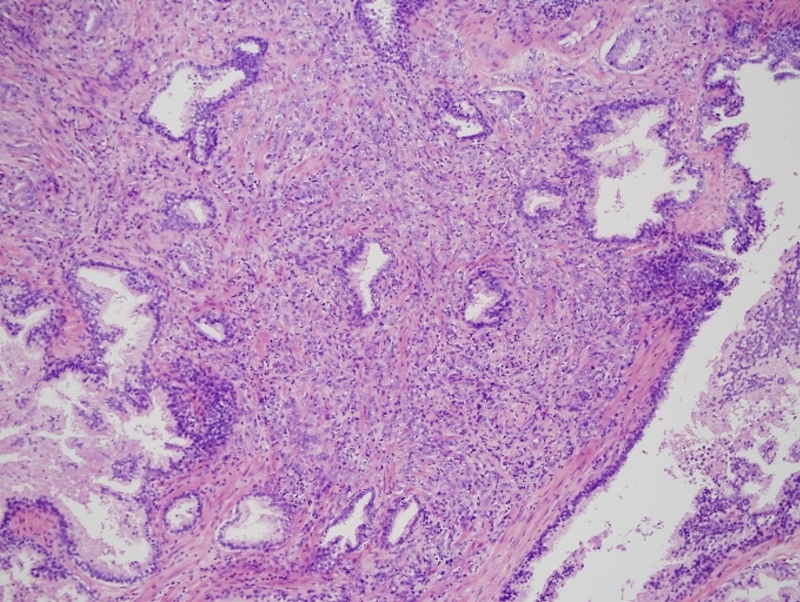
Gleason 4 + 2 |
|
This low power histopthology section using H&E shows a Gleason grade 4+2 (G4-2) revealing irregular cribriform glands. Courtesy Alok Anand MD and Sheema Sheth MD 99416 |
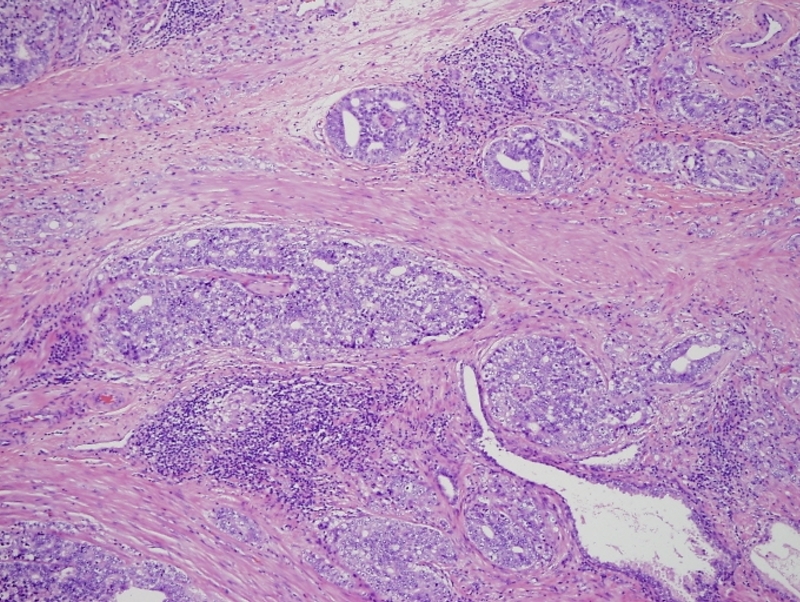
Gleason 5+3 |
|
This low power histopthology section using H&E shows a Gleason 5+3 (G5-3) revealing cells in solid strands infiltrating around normal glands. Courtesy Alok Anand MD and Sheema Sheth MD 99418 |
Imaging
US has not been used as a diagnostic imaging study because of its low sensitivity in the diagnosis of prostate cancer. It is therefore used mainly as a guide for transrectal biopsy.
US guided biopsy
Ultrasound is used in biopsies to ensure that needle placement is positioned in the prostate and specifically in regions of the peripheral zone. Since malignant lesions do not necessarily show abnormal echogenicity the prostate is “randomly” biopsied.. Historically in the early 1990’s when US guided biopsies were introduced the traditional sextant method – 3 cores from either side was the protocol. In view of the high rate of false negatives as well as inconsistencies in pathological diagnosis the pattern is to obtain more tissue at the initial biopsy. It is now usual practice to obtain 5-6 specimens from either side.
Saturation biopsy of the prostate has taken the step one further typically using 24 to 80 cores, with a basis for an intensive search for prostate cancer in high risk patients who may have had multiple negative extended prostate biopsies, or patients with persistently elevated or rising prostate-specific antigen (PSA). It may also be used in patients with prior evidence of atypia, or with biopsies that had shown high-grade prostate intraepithelial neoplasia (PIN) . Even using this procedure false negatives may still occur. A transperineal approach is often selected over a transrectal approach.
The peripheral zone in this instance is divided into medial and lateral aspects and in each of these areas 6 biopsies are taken resulting in a total of 24 cores
One step further is the use of transperineal or transrectal 3D template-guided stereotactic saturation prostate biopsy, typically using 30 to 80 cores where the specimens are taken from separate parts of the prostate and these are mapped. Transperineal biopsy requires anesthesia, and may be asociated with urinary retention. Transrectal biopsy can be domne under local and appears to be the current preferred technique.
Color flow Doppler, power doppler elastography and contrast ultrasound created by microbubble techniques are newer methods of evaluation. In the latter technique isoechoic lesion become hyperechoic with the accumulation of the microbubbles in the lesion.

Lymphoma Not All Masses of the Prostate are Carcinoma or BPH |
|
The CT scan of an 80 year-old man show mass on the left side of the prostate (a,b green arrow) with a displaced non dilated ureter (b yellow arrow) . The findings are non specific but in this case were shown to be lymphoma of the prostate. Image Courtesy Ashley Davidoff MD Copyright 2010 29771cL.8s |
Regional Spread
|
Irregular Shape of Carcinoma as it Invades into Surrounding Tissue |
|
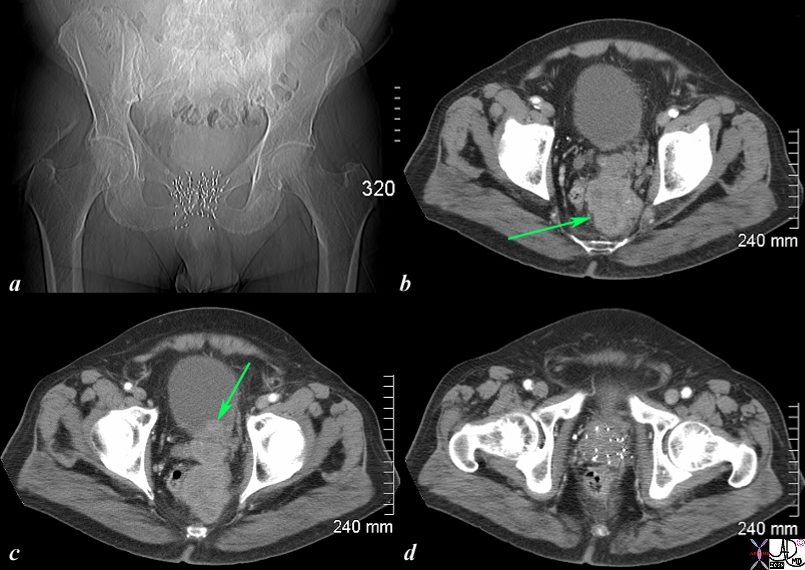
The CT scan of the pelvis is from an 76 year old man with known prostate carcinoma treated with implanted radiation seeds. Image a shows multiple radiation seeds within the prostate gland Image b (light green arrow) shows an irregular heterogeneous mass measuring about 5cms extending above the treated field and progressing posteriorly to the presacral space. . Image c shows the same mass as b extending into the base of the bladder (green arrow). Image d shows multiple prostatic seeds in the prostate gland. These finding are consistent with prostate carcinoma treated with implanted radiation seeds but with the tumor now continuing to grow superiorly beyond the confines of the treatment, and inward to the base of the bladder. . Image Courtesy Ashley Davidoff MD Copyright 2010 98929c01.8s |
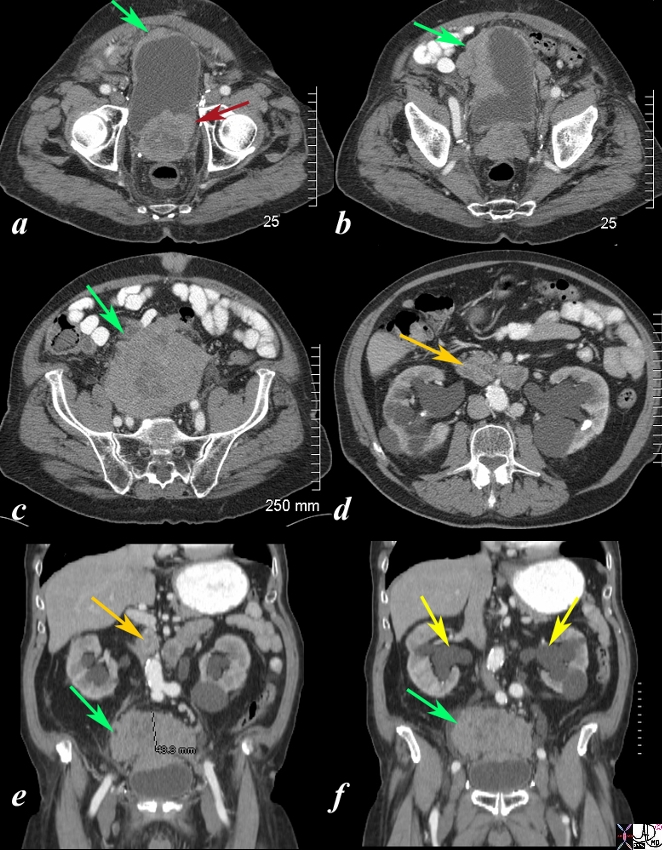
Local Extensive Spread to Bladder and Hydronephrosis |
|
The CT scan is from a 88year old man with known prostate carcinoma. Image a shows multiple the mildly enlarged prostate (maroon arrow) with irregular margins extending to the left of the bladder but with a thickened right anterolateral wall (green arrow). Image b shows extensive irregularity of the superolateral wall which reflects tumor growth superior to and rightward of the bladder (see images below) (green arrow) . . Image c,e,f shows the large mass (green arrow) which has its epicenter above the bladder. Image d and e shows lymphadenoparthy (orange arrows ) anterior to the aorta resulting in hydronephrosis (yellow arrows in f. These findings are consistent with metastatic prostate carcinoma with extensive local extension above the bladder and to retroperitoneal nodes complicated by hydronephrosis. Courtesy Ashley Davidoff MD Copyright 2010 98999cL.81s |
|
Obstructio and Hematuria |
|
The CT scans show an enlarged prostate (a) with hydroureters (b,c yellow arrow) blood-urine level or sediment urine level (red arrow c) with mild hydronephrosis (yellow arrows d) These findings are consistent with obstruction of the kidneys as a result of prostatic enlargement, and in this case prostatic carcinoma. Image Courtesy Ashley Davidoff MD Copyright 2010 18670cL.8s |
Nodal metastases
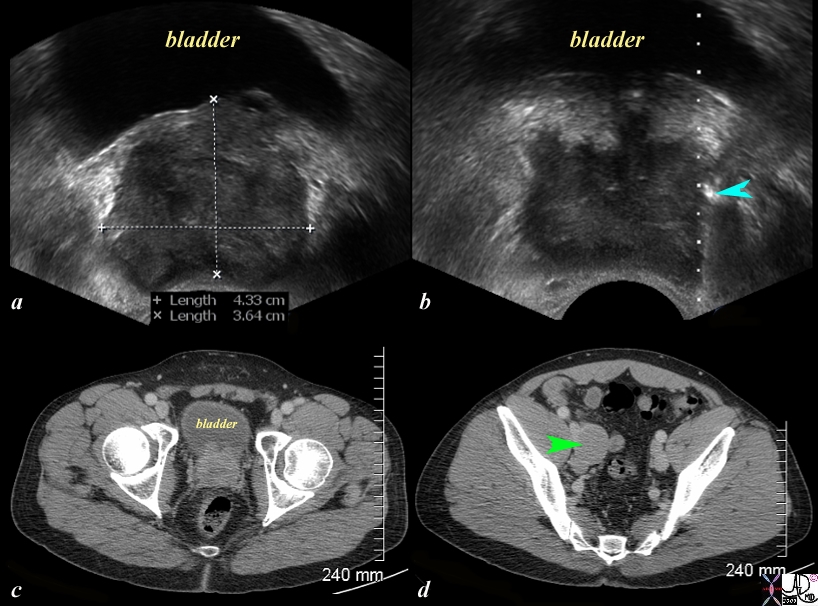
Nodal Metastases |
|
The series of US and CTscans are from a 50 year old man with an elevated PSA Image a is a transverse ultrasound showing a minimally prominent nodular prostate. Image b shows the needle tip (teal blue arrow) in the left peripheral zone. Image c is a transverse image of the prostate showing a nodular anterior surface while image d shows an almost 3cms external iliac node. The biopsy was positive for prostate carcinoma Image Courtesy Ashley Davidoff MD Copyright 2010 97374c.81s |

Local Spread and Iliac Nodes |
|
The CT scan of the pelvis is from a 88 year old man with known prostate carcinoma. Image a and b are through the bladder (yellow) showing the abnormal prostatic gland (green) which is growing toward the left side. A stent (solid white tube traverses the prostate and a second tube with hollow center represents the tip of a Foley catheter in the bladder. The seminal vesicles (pink) are seen posteriorly as symmetric structures except for mild compression of the left by the tumor. Image c and d reveal an almost 2cms left internal iliac node through which the stent passes as well. Its size and participation in the obstrcuction of the left ureter is incriminating. Image Courtesy Ashley Davidoff MD Copyright 2010 25481c01.8s |
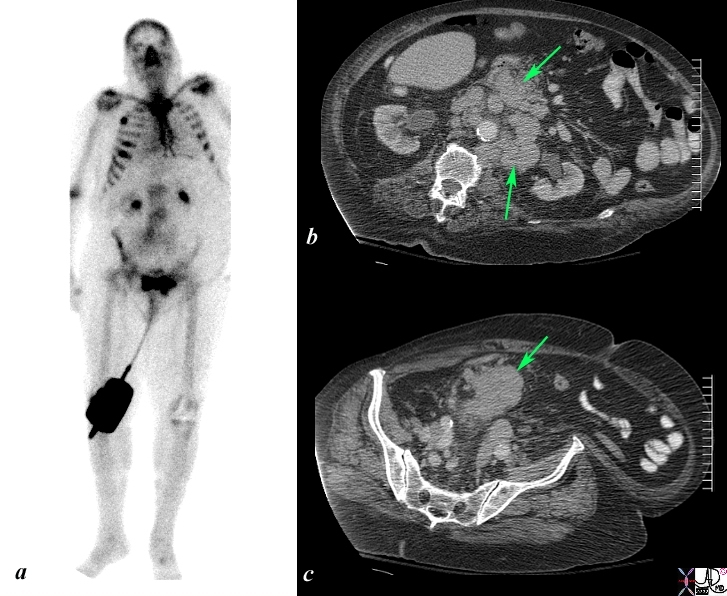
Local Extension and Retroperitoneal Adenopathy |
|
The Bone scan and CT scan is from a 77year old man with known prostate carcinoma. Image a shows multiple hot spots in the ribs consistent with blastic metastatic bone disease. Image b shows extensive lymphadenopathy (green arrows) in the retroperitoneum of the upper abdomen surrounding the ureters resulting in early mild hydronephrosis. . Image c shows a recurrent peritoneal mass (green arrow). These findings are consistent with metastatic prostate carcinoma in the peritoneal cavity, retroperitoneal nodes, and ribs. Image Courtesy Ashley Davidoff MD Copyright 2010 98965c.8s |
Bone Metastases

Ivory Vertebra |
|
The scout film prior to a CTscan of the abdomen and pelvis shows an ivory vertebra (green arrow b) in this patient with known diagnosis of prostate carcinoma. A stent in the right urinary collecting system is likely misplaced and has the proximal portion in the right mid ureter, and distal portion coiled in the ureter. The findings are consistent with blastic metastasis of L2 from prostate carcinoma. Image Courtesy Ashley Davidoff MD Copyright 2010 20615c.8s |
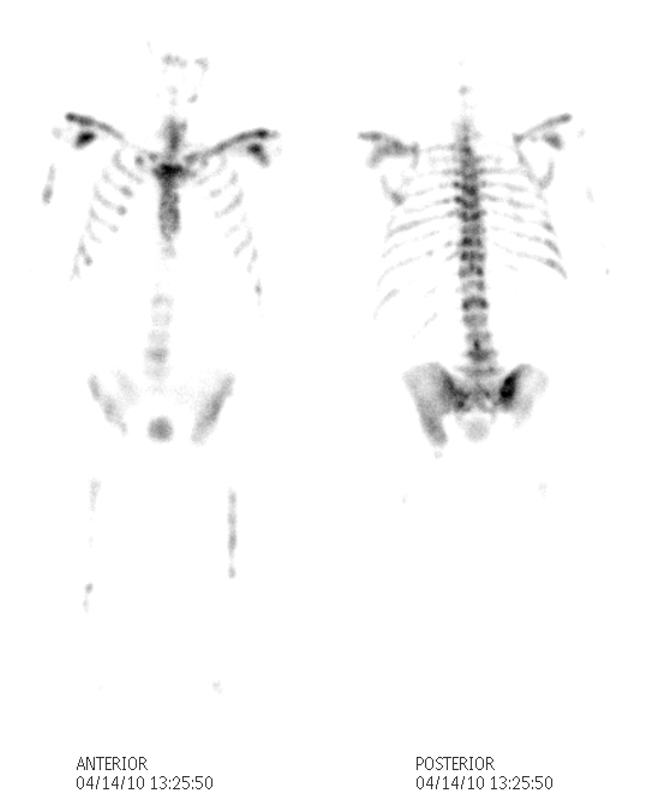
Superscan with Headless Appearance |
|
The patient is a 80 year old man with metastatic prostate carcinoma. His bone scan with 99m technetium labeled diphosphonates shows metastatic disease to vertebral bodies and femurs but more importantly there is an absence of renal and soft tissue activity. The activity of the skull on the posterior view is absent and resulting in a “headless appearance. The scan looks incomplete. These findings are consistent with a “superscan” caused by extensive metastatic disease to the prostate that consumes all the radioactivity and leaves little to be seen in the kidneys or soft tissues. Sometimes the scan can be misinterpreted as normal or technically inadequate by Image Courtesy Ashley Davidoff MD Copyright 2010 98909b.8 |
Bone
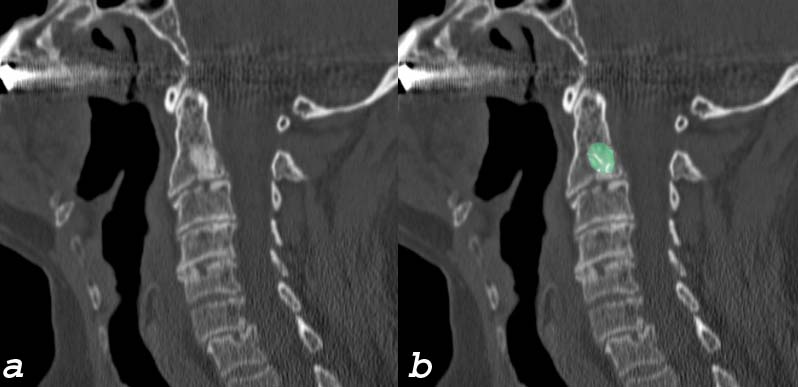
Blastic Metasasis to C2 |
| This elderly man presents with with neck pain bone spine cervical spine c-spine mass blastic mass C2 vertebral body dx blastic metastasis dx probably prostate cancer with metastatic disease CTscan sagittal Courtesy Ashley Davidofff MD 75636c01 |
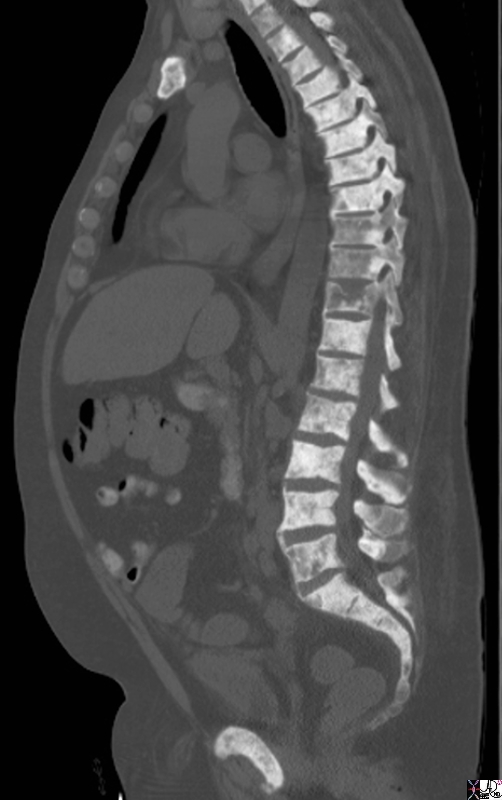
Extensive Blastic Bony Metatsases |
|
The sagittal reconstruction of the thoracic and lumbar spine is from a 50 year old male with known prostate carcinoma shows extensive blastic metastases involving almost all the visualized bones including the entire spine, pubic symphisis and probably sternum. T9 T10 and T11 are involved but not with extensive blastic disease, though T11 shows compression perhaps from associated lytic disease. The findings are consistent with extensive blastic metastasis from primary prostate carcinoma. Image Courtesy Ashley Davidoff MD Copyright 2010 98793.8s |
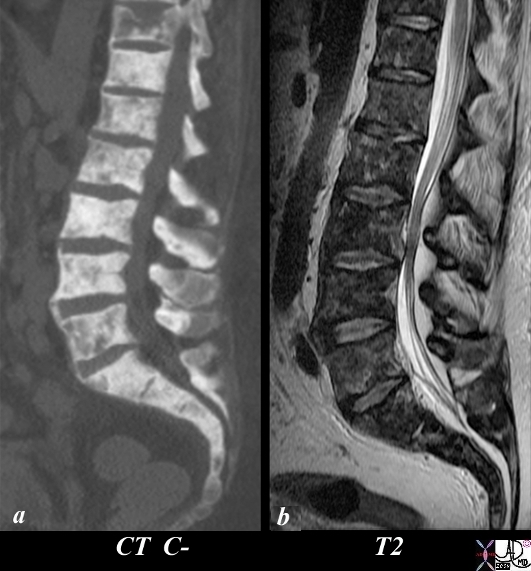
Extensive Bony Metastases CT and MRI |
|
The patient is a 50 year old man with metastatic prostate carcinoma. His lumbar spine with extensive metastatic bone metastases using CT scan (a) and T2 (c) weighted MRI is shown. Image (a) is a CT reconstruction of the lumbar spine and shows extensive blastic metastases involving almost all the visualized bones with lytic and compressive changes in T11. The T2 weighted MRI shows heterogeneous changes within the bone narrow consistent with metastatic disease. The findings are consistent with extensive metastases from primary prostate carcinoma. Image Courtesy Ashley Davidoff MD Copyright 2010 98823cL.81s |
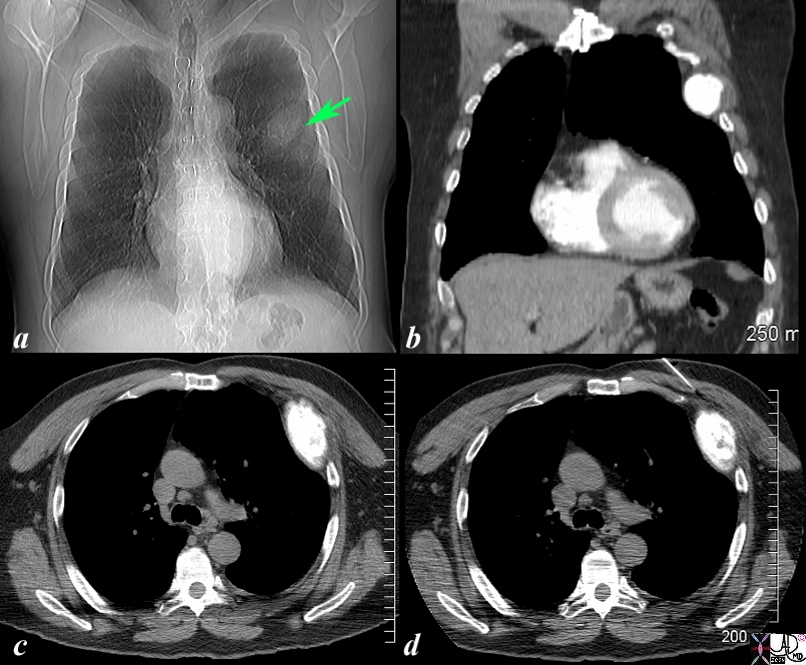
Expansile Blastic Metastasis of a Rib |
|
The scout film of the chest prior to a CTscan shows a vague mass off the left upper chest. (green arrow b) in this 68 year old man with known diagnosis of prostate carcinoma. Image b is a coronal reconstruction of the chest and shows an obvious blastic lesion in the lateral aspect of one of the upper ribs, probably 5. Image c shows the blastic metastasis as an expanded hyperdense area. Image d shows a needle on its way to biopsy the rib which confirmed metastatic prostate carcinoma The findings are consistent with blastic metastasis of a left rib probably 5 from primary prostate carcinoma. Image Courtesy Ashley Davidoff MD Copyright 2010 98787cL.8s |
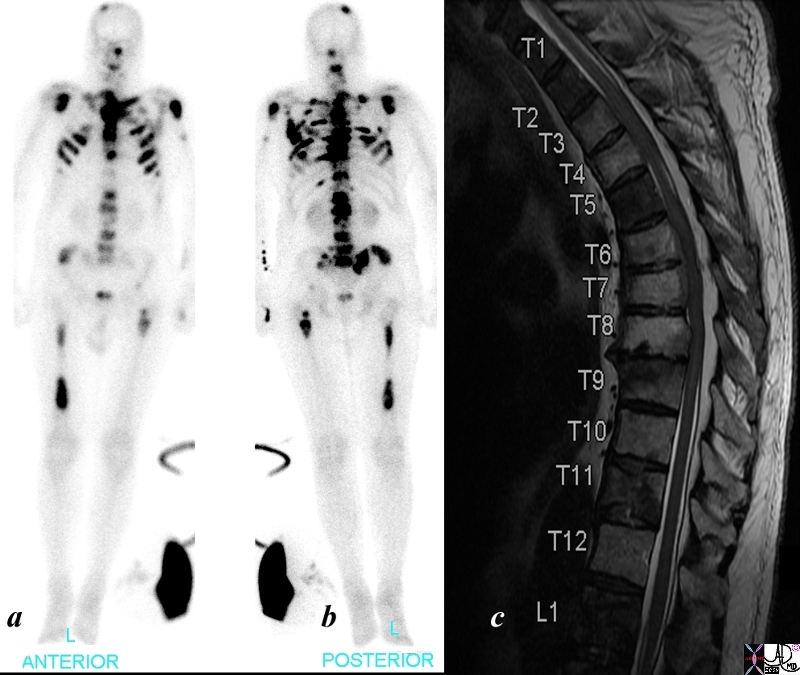
Bone Scan – Almost a Superscan |
|
The patient is a 68 year old man with metastatic prostate carcinoma. His bone scan shows extensive metastatic disease to skull, cervical spine, shoulder joints, sternum, ri thoracic lumbar spine, pelvis and femurs. The T2 weighted image of his thoracic spine show diffuse heterogeneity of the marrow signal indicating extensive metastatic bone metastases using bone scan (a and b) and T2 (c) weighted MRI is shown. The findings are consistent with extensive metastases from primary prostate carcinoma. Image Courtesy Ashley Davidoff MD Copyright 2010 98826cL.8s |
Liver

Liver Metastasis |
|
The core biopsy is from a liver biopsy and shows glandular tissue within the liver consistent with metastatic prostatic adenocarcinoma. Note the pleomorphic hyperchromatic nuclii, with a high nuclear to cytoplasmic ratio consistent with malignancy. Courtesy Ashley Davidoff MD copyright 2009 02682.8s |

Aspiration Cytology Prostate Carcinoma |
|
The aspiration biopsy is from a liver biopsy and shows pleomorphic hyperchromatic nuclii, with a high nuclear to cytoplasmic ratio consistent with malignancy. The patient has a primary prostate carcinoma and metastatic disease from the prostate to the liver is therefore present Courtesy Ashley Davidoff MD copyright 2009 02683.8s |
Lung
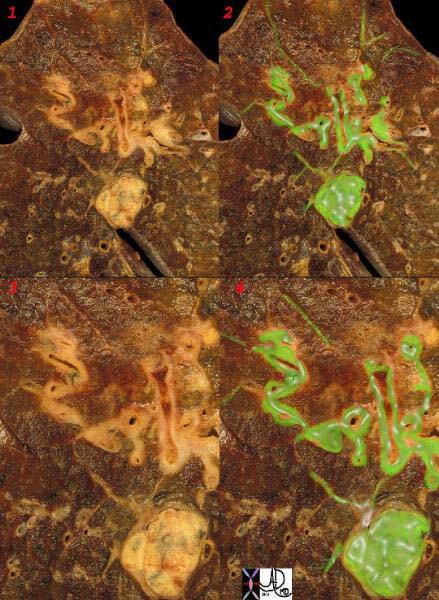
Lymphangitic Spread to The Lungs |
|
This is a post mortem specimen of a lung with lymphangitic spread of prostate carcinoma. Malignant disease is overlaid in green. Note the rounded mass of lymphadenopathy with linear extensions along the thickened bronchovascular bundles. The fine linear bands peripherally are lymphatics congested with tumor. Courtesy Ashley Davidoff MD. 32199cw |
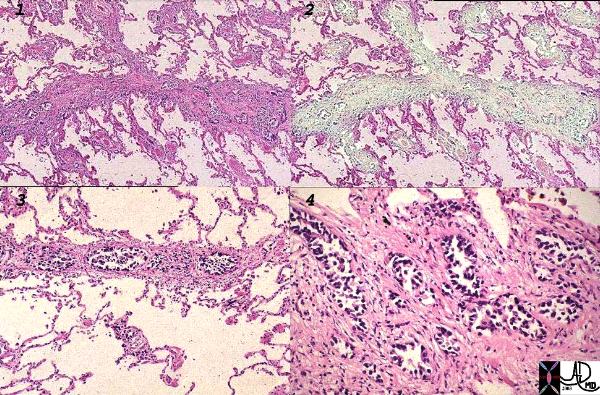
Lymphangitis Obliterans Prostate Carcinoma |
| This histological section is from a patient with protate cancer metastatic to the lymphatics of the lungs – lymphangitis obliterans. The first image shows thickened lymphatics which run in the interlobular septa, overlaid in green in (2). Images 3, and 4 are higher power showing clusters of glandular metatstatic deposits.
Image Courtesy Armando Fraire MD 32226c1 |
Paraneoplastic Syndromes
Up to 8% of patients with cancer are estimated to be affected by paraneoplastic syndromes. Paraneoplastic syndrome is rare with prostatic malignancy, and manifests with endocrine or aberrant immune disorder and is usually seen in patients with histopathology that shows neuroendocrine features or in patients with small cell carcinoma of the prostate. The entitiy is usaly seen in patients with metatstatic diseaseIt is the second most common urological malignancy to be associated with a paraneoplastic syndrome second to renal cll carcinoma. The prostate cancer in these individuals tends to be moderately to poorly differentiated disease. In about 70% of patients the syndrome represents the initial presentation of the disease and in the remainig patients it has manifest as a sign as progression of the disease. The PSA is usually high in these patients. treatment is generally with androgen deprivation therapy (ADT) and treatment of the underlying disease.
Inappropriate Antidiuretic Hormone Syndrome (SIADH) is one of the syndromes that is associated with prostate cancer. The syndrome is more commonly associated with small cell carcinoma of the bronchus. When associated with prostate cancer the prognosis is poor with mean survival of 1 year. ADH is produced by the posterior pituitary in response to low intravascular volume or increased plasma osmolality. In reponse, ADH acts as a vasoconstrictor and also acts on the distal tubules and collecting ducts to increase water absorbtion. Inappropriate secretion of ADH will therefore result in excessive water retention and hyponatremia, with excess urinary excretion. Clinically the patient presents with nausea, vomitting, altered mental status and seizures. Treatnment requires fluid restriction and administration of hypertonic saline.
Cushings syndrome is caused by excess cortisol usually secondary to an inappropriate production of of adrenocorticotrophic hormone (ACTH), precursores of ACTH or corticotrophic releasing hormone. The unchecked stimulation of cortisol production by the adrenal gland leads to both an invcrase in cortisol as well as androgen production. The clinical consequence includes classical Cushingoid features such as central obesity, striae, bruising and proximal muscle weakness. Biochemically a diabtes type response to glucose tolerance test and hypokalemic alkalosis may be seen. The diagnosis is made by high serum cortisol or high urinary cortisol in a 24 hour collection, and confirmed by dexamethasone suppresion test. In the patient who has had androgen deprivation therapy and who has threfore been biochemically castrated, the presence of testosterone. Treatment is with ketoconazole which is an adrenal steroid synthesis inhibitor, and the panCYP17a1 inhibitor abiratonemore specifically inhibits androgen production. Bilateral adrenalectomy is another therapeutic option.
Hypercalcemia is a rare component of the paraneoplastic syndromes of prostate cancer but is not uncommon in the general vista of paraneoplastic syndromes. It is caused by the ectopic production of parathyroid hormone related peptide (PTHRP) which stimulates bone resorption and the osteoclastic activity causes calcium to be released . However because of the nature of prostatic bone metstases to be of a blastic nature they in general will absorb the calcium. This interaction is complex and confounding but in general the serum calcium in patients with ectopic production of PTHRP is normal despite the elevated levels of elevated PTH.
Hematological disorders include the range from hypercoaguability to bleeding diatheses. Both acute and chronic DIC accompany prostate carcinoma as a paraneoplastic syndrome. The diagnosis is made by identifying reduced levels of fibrinogen, thrombocytopenia, elevated D dimersand increased PT and PTT. treatment is directed to managing the prostate cancer as well as directed treatment of the DIC, replacing blood products that are being consumed.
Skin conditions are quite uncommon in the paraneoplastic syndromes associated with prostate cancer, but include dermatomyositis. The skin conditions usually resolve with successful treatment of the prostate cancer.
Neurological syndromes are thought to be caused by immune related dysfunction as a result of the production of tumoral expression of neuronal antigens and the body responds by producing neuronal antibodies which attack dendritic cells in the peripheral and central nervous systems. The result incorporates both sensory and motor dysfunction in the peripheral nervous system with peripheral neuropathyas well as disorders of the temporal lobe an cerebellar dysfuction . The diagnosis is confirmed by identifying antibodies in the serum or CSF while MRI and PET scanning may reveal the structural changes.
Prostatectomy
The first prostatectomy was performed by Hugh Hampton Young in 1904, however due to the common complications of impotence and incontinence the procedure was not popular. As knowledge and technology improved so did the outcomes of surgery. This is the treatment of choice in men with organ confined disease and a life expectancy of more than 10 years.
There are four main ways to perform a radical prostatectomy.
1) Radical retropubic prostatectomy
2) laparoscopic radical prostatectomy
3) robotic assisted laparoscopic prostatectomy
4) Radical perineal prostatectomy
Aim:
To remove the prostate and acheive local control of prostate cancer, including removal of fascia and at times the neurovascular bundle. Also important, is to complete a lymph node dissection.
Procedure:
Most surgeons prefer the retropubic approach to prostatectomy as it allows access to the pelvic lymph nodes for dissection. Also it allows for the prostate to be removed with the surrounding fascia. The procedure is performed through and infraumbilical incision. First the lymph node dissection takes place in the obturator space with the lateral margin of dissection being the pelvic wall and external iliac vein, the distal margin being the node of Cloquet and the proximal margin being the hypogastric vessels. The posterior margin is the obturator nerve within the pelvis. Next, the endopelvic fascia is defined by removing any fat from this structure, and a sharp incision is made in the endopelvic fascia just lateral to the capsular veins of the prostate. Now the prostate is able to be moved laterally. Next, attention is paid to the dorsal venous complex where sutures are placed to control bleeding. Next, the fascia covering the prostate is divided sharply, including division of the puboprostatic ligaments. The dorsal vein complex is then entered and oversewn. Next, the striated urethral sphincter and urethra are then divided just distal to the apex of the prostate. Care is taken to preserve urethral spinchter length. Next, the lateral prostatic fascia is divided just above the neurovascular bundle at the posterolateral level of the prostate, and the prostate is released from the neurovascular bundle starting at the apical level. The posterior prostate is then dissected from the rectum and Denonvilliers’ fascia is then incised just medial to the neurovascular bundle, freeing the bundle from the prostate. After the prostate is mobilized laterally, Denonvilliers’ fascia is incised over the level of the seminal vesicles allowing dissection along the seminal vesicles up laterally over the bladder. Next, the bladder is entered with care taken to avoid entry into the prostate. A midline dissection is then performed to isolate the vas deferens which are clipped and divided. The prostate can then be removed. Next the bladder is attached to the urethra with circumferential sutures in a tension free anastomosis.
Complications:
The major risk of the open prostatectomy are bleeding, incontinence, and impotence. Care with ligation of the dorsal venous complex will assist in decreasing blood loss. Incontinence and impotence rely heavily on preserving the neurovascular bundle.
Prep:
Most surgeons wait 6 weeks after prostatic biopsy to perform a prostatectomy to allow for decrease in inflammation. Patient may be asked to be on a clear liquid diet prior to surgery and perform a bowel prep the day before.
Advantages of laparoscopic or robotic prostatectomy:
The major advantages of these procedures are lower blood loss, decreased pain, and shorter hospital stay.
Androgen Deprivation Therapy
Prostate cancer grows in response to testosterone. The goal of androgen deprivation is to reduce the amount of testosterone that hormone sensitive cancer cells are exposed to. Androgen deprivation therapy is used to control symptoms and slow the rate of growth of metastatic disease. There are three main ways to achieve this:
1) castration: simple orchiectomy is easy, inexpensive, well-tolerated, and almost free of complications. Issues around body image are of greatest concern with this option
2) LHRH agonist: These agents stimulate LH release causing an initial flare of serum testosterone followed by a drop to castrate levels after receptor downregulation. Examples in this drug class include goserelin acetate, leuprolide acetate, triptorelin pamoate.
3) Antiandrogens: these medications block the dihydrotestosterone receptor they are not as effective as castration or LHRH therapy. They do not lower serum testosterone so they generally do not cause a loss of libido or impotence. Medications in this class include biclutamide, flutamide, megestrol, nilutamide.
XRT
|
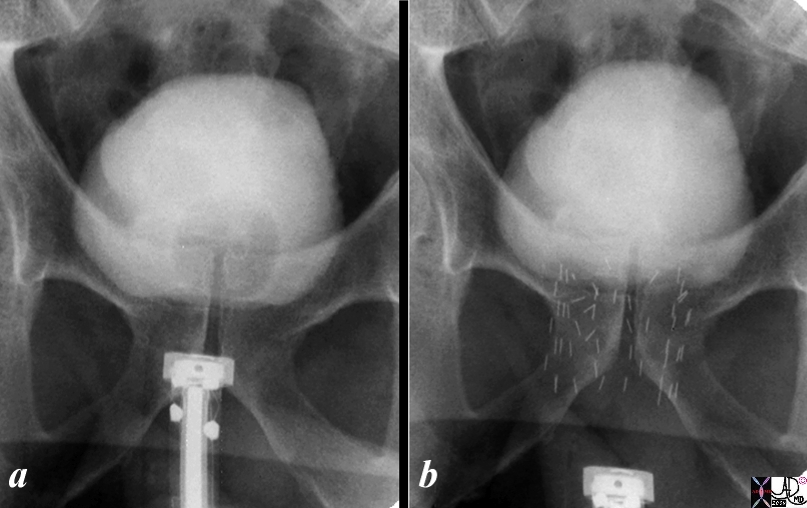
Introduction of the Seeds |
|
The series shows the procedure involved in delivering radiation seeds to the prostate. Image shows the hardware required to introduce the seeds and image b shows the deployed radiation seeds in the prostate. In both a and b the bladder is distended with contrast. The balloon of a Foley can be identified in the bladder in a and has been removed in b. Image Courtesy Ashley Davidoff MD C0pyright 2010 99343c.8s |
Complications
|
Radiation Seeds Embolised to the Lungs |
|
56 year old male with prostate carcinoma has radiation seeds implanted into the prostate gland as seen by the radioopaque pellets in the prostate gland in image a. A chest X-ray following the procedure shows at least 4 of the seeds in the base of the right lung and 2 of the seeds in the mid left lung field overlaid in green (c). Image d is a magnification of the right lower lobe and the seeds are overlaid in green in image e. Courtesy Ashley Davidoff MD copyright 2009 all rights reserved 29676c05.8s |
References
Stamey, T. A. et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 71, 933-938 (1993).
—